CBSE Academic Class XII Chemistry Sample Question Paper 2019-20 : cbseacademic.nic.in
Name of the Board : CBSE Academic
Class : XII STD
Document Type : Sample Question Paper
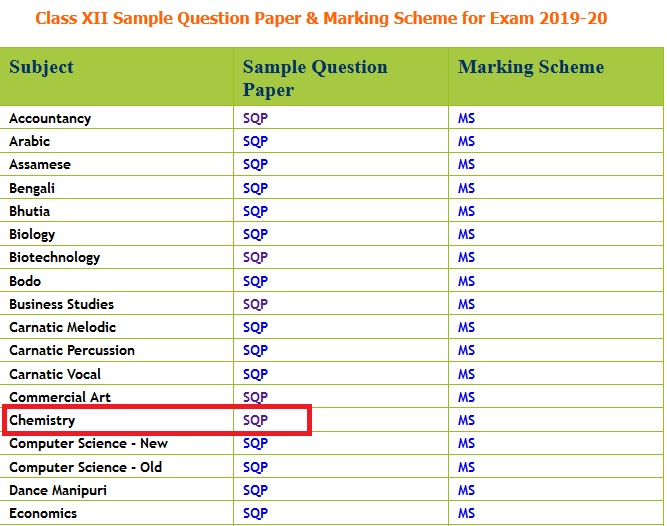
Subject : Chemistry
Paper : Class XII Sample Question Paper 2019-20
Year : 2019-20
Website : http://cbseacademic.nic.in/SQP_CLASSXII_2019_20.html
CBSE XII Chemistry Question Paper
Download Question Paper of Class XII Chemistry Sample Question Paper 2019-20 is now available in the official website of CBSE Academic.
Related : CBSE Class XII Biology Sample Question Paper 2019 : www.pdfquestion.in/34509.html
Section A
1. Read the given passage and answer the questions 1 to 5that follow
A Lead storage battery is the most important type of secondary cell having a lead anode and a grid of lead packed with PbO2 as cathode.
A 38% solution of sulphuric acid is used as electrolyte. (Density=1.294 g mL-1) The battery holds 3.5 L of the acid. During the discharge of the battery, the density of H2SO4 falls to 1.139 g mL-1. (20% H2SO4 by mass)
(1) Write the reaction taking place at the cathode when the battery is in use.
(2) How much electricity in terms of Faraday is required to carry out the reduction of one mole of PbO2?
(3) What is the molarity of sulphuric acid before discharge?
(4) Lead storage battery is considered a secondary cell. Why?
(5) Write the products of electrolysis when dilute sulphuric acid is electrolysed using Platinum electrodes.
Questions 6 to 10 are one word answers :
(6) Name the substance used as depressant in the separation of two sulphide ores in Froth floatation method.
(7) Name the unit formed by the attachment of a base to 1? position of sugar in a nucleoside.
(8) Name the species formed when an aqueous solution of amino acid is dissolved in water?
(9) What type of reaction occurs in the formation of Nylon 6,6 polymer?
(10) Which of the following compoundswould undergo cannizzaro reaction
Benzaldehyde, Cyclohexanone, 2- Methylpentanal.
Questions 11 to 15 are multiple choice questions :
(11) The IUPAC name of the compound shown below is
(a) 2-bromo-6-chlorocyclohex-1-ene
(b) 6-bromo-2-chlorocyclohexene
(c) 3-bromo-1-chlorocyclohexene
(d) 1-bromo-3-chlorocyclohexene
(12) The incorrect statement about LDP is
(a) It is obtained through the free radical addition of ethene.
(b) It consists of linear molecules.
(c) It is obtained by the H-atom abstraction.
(d) Peroxide is used as an initiator.
Questions 16 to 20 :
(A) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(B) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(C) Assertion is correct, but reason is wrong statement.
(D) Assertion is wrong, but reason is correct statement.
16. Assertion : The two strands in double strand helix structure of DNA are complementary to each other
Reason : Disulphide bonds are formed between specific pairs of bases
17. Assertion : Glucose reacts with hydroxylamine to form an oxime and alsoadds a molecule of hydrogen cyanide to give cyanohydrin.
Reason : The carbonyl group is present in the open chain structure of glucose.
18. Assertion : The acidic strength of halogen acids varies in the order HF>HCl>HBr>HI
Reason : The bond dissociation enthalpy of halogen acids decreases in the order HF>HCl>HBr>HI
19. Assertion : C2H5OH is a weaker base than phenol but is a stronger nucleophile than phenol. (1)
Reason : In phenol the lone pair of electrons on oxygen is withdrawn towards the ring due to resonance.
20. Assertion : Aryl halides undergo nucleophilic substitution reactions with ease.
Reason :The carbon halogen bond in aryl halides has partial double bonds character.
Section B
21. Calculate the number of lone pairs on central atom in the following molecule and predict the geometry. XeF4
22. What is meant by Vapour phase refining? Write any one example of the process which illustrates this technique, giving the chemical equations involved.
OR
Write and explain the reactions involved in the extraction of gold.
23. Which one of the following compounds will undergo hydrolysis at a faster rate by SN1 mechanism? Justify.
Download XII Chemistry Question Paper 2019-20 :
https://www.pdfquestion.in/uploads/pdf2019/34598-Ch.pdf
Section C
24. Calculate the freezing point of a solution containing 0.5 g KCl (Molar mass = 74.5 g/mol) dissolved in 100 g water, assuming KCl to be 92% ionized. Kfof water = 1.86 K kg / mol.
25. For the reaction A + B -> products, the following initial rates were obtained at various given initial concentrations Determine the half-life period.
OR
A first order reaction is 50 % complete in 50 minutes at 300 K and the same reaction is again 50 % complete in 25 minutes at 350 K. Calculate activation energy of the reaction.
26. Account for the following:
a) Moist SO2 decolourises KMnO4 solution.
b) In general interhalogen compounds are more reactive than halogens (except fluorine).
c) Ozone acts as a powerful oxidizing agent
27. Identify the product formed when propan-1-ol is treated with Conc. H2SO4 at 413 K . Write the mechanism involved for the above reaction.
28. (a) Give chemical tests to distinguish between the following pairs of compounds
(i) Ethanal and Propanone.
(ii) Pentan-2-one and Pentan-3-one.
(b) Arrange the following compounds in increasing order of their acid strength
Benzoic acid, 4- Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4- Methoxybenzoic acid.
OR
Compare the reactivity of benzaldehyde and ethanal towards nucleophilic addition reactions. Write the cross aldol condensation product between benzaldehyde and ethanal.
29. Define and write an example for the following
(a) Broad spectrum antibiotics.
(b) Analgesics