Vigyan Dhara Scholarship Admission Test VSAT Class XII Sample Paper : vigyandhara.in
Organisation : Vigyan Dhara
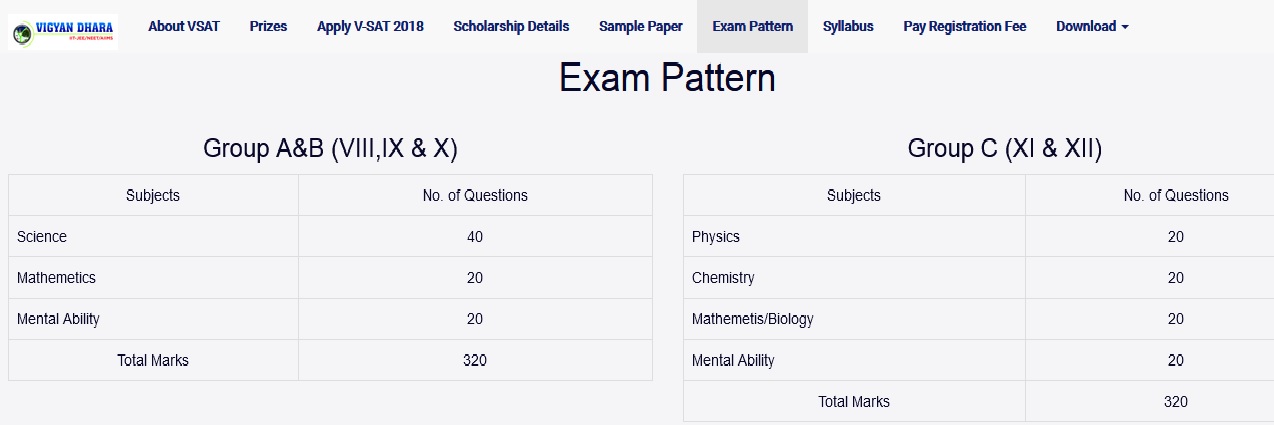
Exam : VSAT – Vigyan Dhara Scholarship Admission Test
Document Type : Sample Paper
Subjects : Physics, Chemistry, Biology/Math, Mental Aptitude
Class : XII Std
Website : vigyandhara [dot] in
VSAT Scholarship Admission Test Class XII Sample Paper
Sample Question Paper of Vigyan Dhara Scholarship Admission Test Class XII Standard for all subjects Question Paper is now available in the official website of Vigyan Dhara
Related / Similar Question Paper :
Vigyan Dhara VSAT Class XI Sample Paper
Important Instructions
1. This booklet contains 50 Questions.
2. All questions are compulsory and carry 2 mark,
3. There will be no negative marking.
4. Immediately fill in the particulars on this page of the Test Booklet with Blue/Black Ball Point Pen.
5. No candidate is allowed to carry any textual material, printed or written, bits of papers, mobile phone, any electronic device, etc
6. Rough work is to be done on the space provided for this purpose in the Test Booklet only
7. On completion of the test, the candidate must hand over the Answer Sheet to the Invigilator on duty in the Room/ Hall; however, the candidates are allowed to take away this Test Booklet with them.
Download Question Paper :
https://www.pdfquestion.in/uploads/VSAT12th.pdf
Model Questions
Physics
1. Electric charges are distributed in a small volume. The flux of the electric field through a spherical surface of radius 10 cm surrounding the total charge is 25 V-m. The flux over a concentric sphere of radius 20 cm will be –
(A) 25 V-m
(B) 50 V-m
(C) 100 V-m
(D) 200 V-m
2. Figure shows a charge q placed at the centre of a hemisphere. Another charge Q can be put on the positions A, B, C and D. In which position(s) of this another charge, the flux of the electric field through the hemisphere remains unchanged –
(A) A, C
(B) B, D
(C) C, D
(D) A, D
3. Five capacitors are connected as shown in figure below. Initially S is opened and all capacitors are uncharged. When S is closed, steady state is obtained. The p.d. between the points M and N will be ……………….
(A) 6 V
(B) 8V
(C) 12V
(D) 4V
4. The equivalent capacitance between point A and B is –
(A) C/4
(B) C/2
(C) C
(D) 2C
5. In the electric circuit shown in figure, the reading of voltmeter V1 is 26 volt, and the reading of ammeter A1 is 2 ampere. The value of resistance x is – (all instruments are ideal)
(A) 2
(B) 4
(C) 6
(D) 8
6. A uniform magnetic field exists in region given by kˆ 5 jˆ 4 iˆ B ? 3 ? . A rod of length 5 m is placed along y-axis is moved along x-axis with constant speed 1 m/sec. Then induced e.m.f. in the rod will be-
(A) zero
(B) 25 volt
(C) 20 volt
(D) 15 volt
7. Consider the situation shown in figure. If the switch is closed and after some time it is opened again, the closed loop will show-S closed loop
(A) an anticlockwise current-pulse
(B) a clockwise current-pulse
(C) an anticlockwise current-pulse and then a clockwise current-pulse
(D) a clockwise current-pulse and then an anticlockwise current-pulse
8. Two identical bulbs B1 and B2 are connected to an ac source. B1 is connected in series with a coil of 100 mH and B2 with a capacitor of 10 ?F as shown in the figure. The brightness of B1 and B2 will be-
(A) Same in both
(B) More in B1
(C) Depending on the frequency of the source
(D) More in B2
Chemistry
21. Consider a Body Centered Cubic(bcc) arrangement, let de, dfd, dbd be the distances between successive atoms located along the edge, the face-diagonal, the body diagonal respectively in a unit cell.Their order is given by:
(A) de < dfd < dbd
(B) dfd > dbd > de
(C) dfd > de > dbd
(D) dbd > de > dfd,
23. The maximum percentage of available volume that can be filled in a face centred cubic system by atoms is-
(A) 74%
(B) 68%
(C) 34%
(D) 26%
24. The spinal structure (AB2O4) consists of an fcc array of O2– ions in which the :
(A) A cation occupies one-eighth of the tetrahedral holes and B cation occupies one-half of octahedral holes
(B) A cation occupies one-fourth of the tetrahedral holes and the B cations the octahedral holes
(C) A cation occupies one-eighth of the octahedral hole and the B cation the tetrahedral holes
(D) A cation occupies one-fourth of the octahedral holes and the B cations the tetrahedral holes
25. The vapour pressure of the solution of two liquids A(pº = 80 mm) and B(pº = 120 mm) is found to be 100 mm when xA = 0.4. The result shows that
(A) solution exhibits ideal behaviour
(B) solution shows positive deviations
(C) solution shows negative deviations
(D) solution will show positive deviations for lower concentration and negative deviations for higher concentrations.
26. If Mnormal is the normal molecular mass and ? is the degree of ionization of K3[Fe(CN)6], then the abnormal molecular mass of the complex in the solution will be :
(A) Mnormal (1 + 2?)–1
(B) Mnormal (1 + 3?)–1
(C) Mnormal (1 + ?)–1
(D) equal to Mnormal
27. PtCl4 . 6H2O can exist as a hydrated complex 1 molal aq. solution has depression in freezing point of 3.72°. Assume 100% ionisation and Kf(H2O) = 1.86° mol–1 kg, then complex is –
(A) [Pt(H2O)6]Cl4
(B) [Pt(H2O)4Cl2]Cl2 . 2H2O
(C) [Pt(H2O)3Cl3]Cl . 3H2O
(D) [Pt(H2O)2Cl4] . 4H2O
28. Osmotic pressure of 30% solution of glucose is 1.20 atm and that of 3.42% solution of cane sugar is 2.5 atm. The osmotic pressure of the mixture containing equal volumes of the two solutions will be
(A) 2.5 atm
(B) 3.7 atm
(C) 1.85 atm
(D) 1.3 atm.
29 Which is not true for a second order reaction ?
(A) It can have rate constant 1 × 10–2 L mol–1 s–1
(B) Its half-life is inversely proportional to its initial concentration
(C) Time to complete 75% reaction is twice of half-life
30. MnO4 – + 8H+ + 5e– ??? Mn2+ + 4H2O, If H+ concentration is decreased from 1 M to 10–4 M at 25ºC, where as concentration of Mn2+ and MnO4 – remain 1 M.
(A) the potential decreases by 0.38 V with decrease in oxidising power
(B) the potential increases by 0.38 V with increase in oxidising power
(C) the potential decreases by 0.25 V with decrease in oxidising power
(D) the potential decreases by 0.38 V without affecting oxidising power
31. If 0.224 L of H2 gas is formed at the cathode, the volume of O2 gas formed at the anode under identical conditions, is
(A) 0.224 L
(B) 0.448 L
(C) 0.112 L
(D) 1.12 L
37. Finely divided catalyst has greater surface area and has greater catalytic activity than the compact solid. If a total surface area of 6291456 cm2 is required for adsorption in a catalysed gaseous reaction, then how many splits should be made to a cube of exactly 1 cm in length to achieve required surface area. (Given : One split of a cube gives eight cubes of same size)
(A) 60
(B) 80
(C) 20
(D) 22
38. Which one is false in the following statement ?
(A) A catalyst is specific in its action
(B) A very small amount of the catalyst alters the rate of a reaction
(C) The number of free vacancies on the surface of the catalyst increases on sub-division
(D) Ni is used as a catalyst in the manufacture of ammonia
39. For the coagulation of 200 mL of As2S3 solution, 10 mL of 1 M NaCl is required. What is the coagulating value (number of milli moles of solute needed for coagulation of 1 liter of solution) of NaCl.
(A) 200
(B) 100
(C) 50
(D) 25
40. Peptisation is
(A) conversion of a colloidal into precipitate form
(B) conversion of precipitate into colloidal sol
(C) conversion of metal into colloidal sol by passage of electric current
(D) conversion of colloidal sol into macromolecules
Biology
41. The coconut water from tender coconut represents
(A) Free nuclear endosperm
(B) Endocarp
(C) Fleshy mesocarp
(D) Free nuclear proembryo
42. Seed formation without fertilization in flowering plants involves the process of
(A) Apomixis
(B) Sporulation
(C) Budding
(D) Somatic hybridization
43. Filiform apparatus is a characteristic feature of:
(A) Zygote
(B) Suspensor
(C) Egg
(D) Synergid
44. A human female with Turner’s syndrome:
(A) Has 45 chromosomes with XO
(B) Has one additional X chromosome
(C) Exhibits male characters
(D) Is able to produce children with normal husband
45. ABO blood grouping is controlled by gene I which has three alleles and show co-dominance. There are six genotypes. How many phenotypes in all are possible?
(A) Six
(B) Three
(C) Four
(D) Five
46. If a colourblind woman marries a normal visioned man, their sons will be
(A) All normal visioned
(B) One-half colourblind and one-half normal
(C) Three-fourths colourblind and one-fourth normal
(D) All colourblind
47. Synthesis of leading and lagging strand require
(A) Single primer
(B) Single and many primers respectively
(C) Many and single primers respectively
(D) Many primers
48. Identification and binding of RNA polymerase to the promoter sequence is a function of
(A) Rho factor
(B) Sigma factor
(C) Beta factor
(D) Omega factor
49. Removal of RNA polymerase III from nucleoplasm will affect the synthesis of
(A) mRNA
(B) rRNA
(C) tRNA
(D) hnRNA
50. Removal of introns and joining of exons in a defined order during transcription is called
(A) Slicing
(B) Splicing
(C) Looping
(D) Inducing