Matrix Talent Search Exam MTSE Class XI Sample Paper : matrixmtse.in
Name of the Organization : Matrix JEE Academy
Exam : MTSE – Matrix Talent Search Exam
Document Type : Sample Papers
Category or Subject : Class XI
Website : http://www.matrixmtse.in/mtse-sample-papers.php
Download Sample Question Paper : https://www.pdfquestion.in/uploads/23819-MTSE11th.pdf
Matrix Talent Search Exam XI Sample Paper
** MTSE not only focuses on student’s knowledge, it has two separate papers to assess students knowledge as well as inherent potential.
Related : Matrix Talent Search Exam MTSE Class X Sample Question Paper : www.pdfquestion.in/23815.html
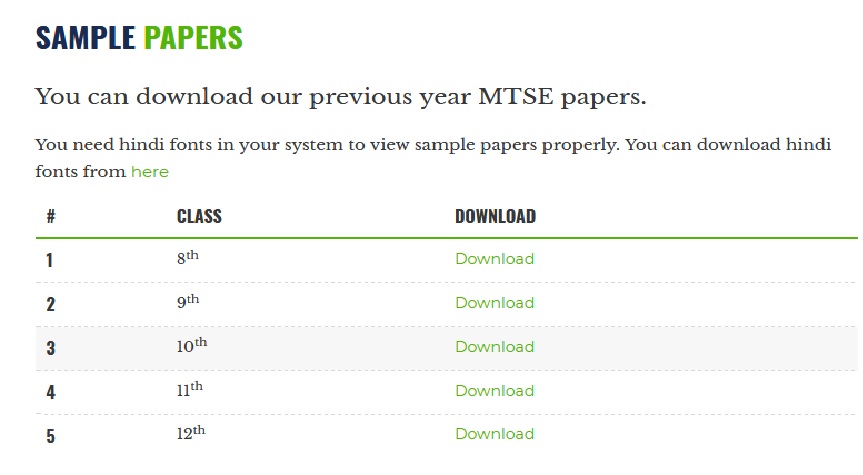
** You can download our previous year MTSE papers. You need hindi fonts in your system to view sample papers properly. You can download hindi fonts from here
Instructions
1. All questions are Single correct type questions. Each of these questions has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct. For each question, you will be awarded 4 marks if you have darkened only the bubble corresponding to the correct answer and zero mark if no bubble are darkened. In all other cases, no negative mark will be awarded.
2. Indicate the correct answer for each question by filling appropriate bubble in your answer sheet.
3. Use of Calculator, Log Table, Slide Rule and Mobile is not allowed.
Useful Data :
Atomic weights: Al = 27, Mg = 24, Cu = 63.5, Mn = 55, Cl = 35.5, O = 16, H = 1, P = 31, Ag = 108, N = 14, Li = 7, I = 127, Cr = 52, K=39, S = 32, Na = 23, C = 12, Br = 80, Fe = 56, Ca = 40, Zn = 65.5, Ti = 48, Ba = 137, U = 238, Co= 59, B =11, F = 19, He = 4, Ne = 20, Ar = 40 , Mo = 96, Ni = 58.5, Sr = 87.5, Hg = 200.5 , Tl = 204, Pb = 207 [Take : ln 2 = 0.69, ln 3 = 1.09, e = 1.6 × 10–19, me= 9.1 × 10–31 kg ] Take g = 10 m/s2 unless otherwise stated MTSE for Class 8th
MTSE for Class XI
Single Correct Choice Type
Q.1 to Q.10 has four choices (A), (B), (C), (D) out of which ONLY ONE is correct.
Section I – Physics
1. The distance and displacement of a moving object are definitely equal when it
(A) Moves in a circle
(B) Slows down
(C) Speeds up
(D) Moves straight without turning back
2. A net acceleration of 4 m/s2 acts on a 5 kg mass kept at rest. Distance travelled by the mass in 6 s is
(A) 72 m
(B) 36 m
(C) 24 m
(D) 12 m
3. An object of mass 5 kg occupies a volume of 500 cm3. The relative density of the mass with respect to water is
(A) 0.1
(B) 10
(C) 0.01
(D) 1
4. A ball of mass 500 g kept near the earth’s surface will attract the earth with a force of
(A) 9.8 N
(B) 19.6 N
(C) 4.9 N
(D) 29.4 N
5. Friction between two surfaces in contact increases when
(A) A layer of grease is applied between them
(B) They are pressed harder against each other
(C) They move over each other
(D) They are pulled apart
6. Write down the unit of Universal Gravitational constant ‘G’ in S.I.
(A) Nm2 /kg2
(B) Newton
(C) kg2/Nm2
(D) dyne × cm2/g2
7. When a running motorbike accelerates suddenly, the pillion rider has a tendency to fall backward. This is an example of
(A) Newton’s first law of motion
(B) Newton’s second law of motion
(C) Newton’s third law of motion
(D) Newton’s law of gravitation
8. If the time-displacement graph of a particle is parallel to the time-axis, then velocity of the particle is :-
(A) infinity
(B) unity
(C) equal to acceleration of the body
(D) zero
10. A force of 6 kg and another of 8 kg can be applied together to produce the effect of a single force of-
(A) 1kg
(B) 11kg
(C) 15 kg
(D) 20 kg
Section II – Chemistry
11. One mole of CO2 contains
(A) 6.02 X 1023 atoms of O
(B) 6.02 X 1023 atoms of Carbon
(C) 18.10 X 1023 molecules of CO2
(D) 3 gm atoms of CO2
12. An atom is
(A) the smallest particle of matter known
(B) the smallest particle of a gas
(C) the smallest indivisible particle of an element that can take part in a chemical change
(D) radioactive emission
13. Acid + Metal-oxide ? ?
(A) Base + Water
(B) Salt + Water
(C) Base + Salt
(D) Metal + Salt
14. Number of electrons in one mole of hydrogen is
(A) 6.02 X 1023
(B) 12.046 X 1023
(C) 3.0115 X 1023
(D) indefinite
16. Pentane has the molecular formula C5H12. It has
(A) 5 covalent bonds
(B) 12 covalent bonds
(C) 16 covalent bonds
(D) 17 covalent bonds
17. Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of :
(A) helium
(B) neon
(C) argon
(D) krypton
18. Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic Table :
(A) The elements become less metallic in nature
(B) The number of valence electrons increases.
(C) The atoms lose their electrons more easily
(D) The oxides become more acidic
19. Which of the folowing is Dobereiner’s triad :
(A) Ne, Ca, Na
(B) H2, N2, O2
(C) Li, Na, K
(D) Na, Br, Ar
20. A solution turns blue litmus red. Its pH value is likely to be :
(A) 4
(B) 7
(C) 9
(D) 12
Section III – Maths
21. The roots of Quadratic equation x2 + 14x + 45 = 0 are –
(A) – 9,5
(B) 5, 9
(C) – 5, 9
(D) – 5, – 9
22. The roots of the equation x2 – x – 3 = 0 arelehdj.
(A) Imaginary
(B) Rational
(C) Irrational
(D) None of these
23. If the roots of the equation x2 – 10 x + 21 = m are equal then m is –
(A) 4
(B) 25
(C) – 4
(D) 0
24. Which term of the series 3 + 8 + 13 + 18 + … is 498-
(A) 95th
(B) 100th
(C) 102th
(D) 101th
25. The number of terms in the series 101 + 99 + 97 + …..+ 47 is-
(A) 25
(B) 28
(C) 30
(D) 20
26. The 19th term from the end of the series2 + 6 + 10 + ….+ 86 is –
(A) 6
(B) 18
(C) 14
(D) 10
27 A = {a, e, i, o, u}and B = {i, o} then the true statement is –
(A) A + B
(B) B – A
(C) A * B
(D) A is equivalent B
28. Given the sets A = {1,2,3}, B = {3,4}, C = {4,5,6}, then A ?(B ? C) is –
(A) {3}
(B) {1,2,3,4}
(C) {1,2,4,5}
(D) {1,2,3,4,5,6}
29. The number of ways in which 3 persons can occupy 6 rooms separately is-
(A) 2
(B) 20
(C) 120
(D) 216
30. If nCn–4= 5 then the value of n is –
(A) 5
(B) 3
(C) 4
(D) 6
Syllabus For Class 11Th
Physics : 100% 10th std. + 50% 11th
Chemistry : 100% 10th std. + 50% 11th
Maths : 100% 10th std. + 50% 11th
Very Important :
A. The question paper consists of 3 parts (Physics, Chemistry & Mathematics ). Please fill the OMR answer
B. Please ensure that the Question Paper you have received contains All the questions in each Section and Pages. If you found some mistake like missing questions or pages then contact immediately to the Invigilator.